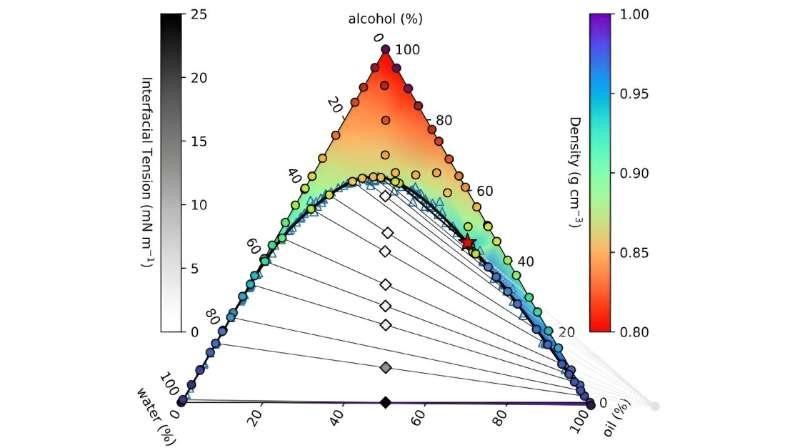
The ouzo phase diagram. The full figure legend can be found in the corresponding journal paper.
Mathematicians at Loughborough University have turned their attention to a fascinating observation that has intrigued scientists and cocktail enthusiasts alike: the mysterious way ouzo, a popular anise-flavored liquor, turns cloudy when water is added.
The researchers’ exploration of this seemingly simple phenomenon, known as the “Ouzo Effect,” has resulted in a new mathematical model that offers insights into the spontaneous formation of microscopic droplets and how they can remain suspended in a liquid for a long time.
Revealing the math taking place in the glass could have far-reaching implications beyond the world of beverages, such as the creation of new materials.
“Ouzo is essentially three things: alcohol, anise oil, and water,” explains Dr. David Sibley, an expert in mathematical modeling.
“When water is added, microscopic droplets form that are made mostly of oil, and these are a result of the anise oil separating from the alcohol-water mixture. This causes the drink to turn cloudy as the droplets scatter light.”
He continued, “This emulsification—the suspension of well-mixed oil droplets in the liquid—is something that requires a lot of energy in other systems and foods. For example, food emulsions such as mayonnaise and salad dressings require vigorous whisking to achieve a smooth and stable mixture. For ouzo, however, the emulsification happens spontaneously.
“What’s also surprising is how long these droplets, and the resulting cloudiness, remain stable in the mixture without separating, especially when compared to other food emulsions. If you’ve ever made an olive oil and balsamic vinegar dressing, you’ll notice that the two liquids start to separate after a short time, requiring more whisking to bring them back together. The ouzo-water emulsion remains stable for a much longer period.
“Understanding how and why this happens in ouzo could lead to the development of new materials, especially in fields such as in pharmaceuticals, cosmetics, and food products, where the stability and distribution of microscopic particles are critical.”
The Loughborough researchers, in collaboration with experts from the University of Edinburgh and Nottingham Trent University, have uncovered the mathematical principles that explain how the droplets and surrounding liquid—two distinct ‘phases’ within the mixture—form and can remain stable together for long periods.
By mixing alcohol, oil, and water in varying proportions, they were able to observe phase separation and measure key properties like surface tension.
They used this data and a statistical mechanical modeling method known as ‘classical density functional theory’ to develop their mathematical model.
This model has been used to calculate a phase diagram that details the stable combinations of the ouzo ingredients.
The research has been published in the journal Soft Matter and is featured on the front cover of the latest issue. The paper is titled “Experimental and theoretical bulk phase diagram and interfacial tension of ouzo.”
“You could say, what looks cloudy is now clearer,” said Professor Andrew Archer, the first author of the journal paper.
“What is also fun is that simple models like this can predict a lot—similar to recent, parallel research we did that reveals how long droplets we sneeze into the air can persist.
“As is often the case, ‘blue skies’ fundamental research can say something profound about an experience that occurs in regular life—like serving and drinking ouzo.”
For more such insights, log into our website https://international-maths-challenge.com
Credit of the article to be given Meg Cox, Loughborough University